Abstract
Background: In sickle cell disease (SCD), a single nucleotide change in the β-globin gene coding sequence results in the production of hemoglobin S (HbS), which polymerizes when deoxygenated and leads to red blood cell (RBC) sickling, hemolysis, anemia, diminished microvascular blood flow, vascular endothelial cell activation, vaso-occlusion, and ischemia. Pharmacological increase of the oxygenated HbS concentration in RBCs has been shown to be a well-tolerated and effective approach to inhibiting HbS polymerization, reducing RBC sickling, and improving oxygen (O2) delivery by reducing anemia and improving blood rheology. GBT021601, a potent second-generation small-molecule HbS polymerization inhibitor, is currently under investigation and has previously been shown to increase hemoglobin (Hb), improve hemolysis, and improve RBC health in a murine model. However, little is known regarding its impact on vaso-occlusion and microvascular dysfunction.
Objective: To examine the effects of GBT021601 on SCD-induced microvascular dysfunction, capillary perfusion and density, Hb-O2 oxygen affinity, and Hb and hematocrit levels in a murine model of SCD.
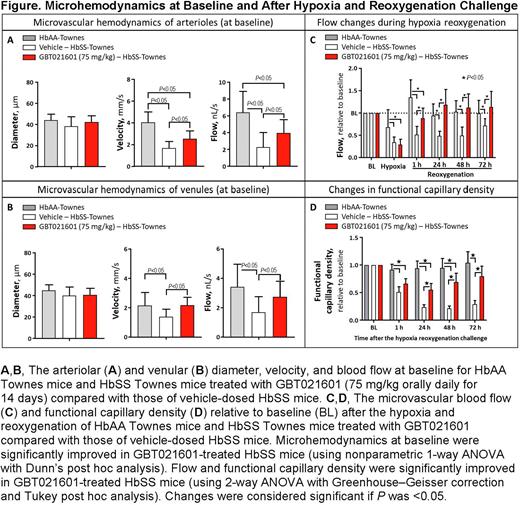
Methods: Capillary perfusion was measured by functional capillary density (FCD, a marker of capillary perfusion defined as the number of capillaries with RBCs in transit) in a murine model of SCD with an implanted dorsal window chamber (Figure). HbSS Townes mice were treated with GBT021601 (75 mg/kg) by oral gavage daily for 14 days and were compared with untreated HbSS and HbAA Townes mice.
Results: Chronic treatment of HbSS mice with GBT021601 led to increased Hb-O2 affinity, evidenced by a reduction in blood p50 (partial O2 pressure at which Hb is 50% saturated with O2) from 32 mm Hg to 14 mm Hg. GBT021601 significantly reduced anemia by increasing total Hb and hematocrit from 8.3 g/dL and 26% in untreated HbSS mice to 11.2 g/dL and 37% in GBT021601-treated HbSS mice, respectively (P<0.01). Moreover, GBT021601 treatment prevented microvascular dysfunction (reduced RBC velocities and blood flow) in the arterioles and venules of HbSS mice.
Increased capillary density is indicative of greater oxygen transport. Challenging HbSS mice with hypoxia (8% O2 for 1 hour) followed by reoxygenation (21% O2) induced vaso-occlusion, causing reduced microvascular blood flow and FCD in untreated mice. However, in GBT021601-treated HbSS mice, microvascular blood flow and FCD were significantly higher than those in untreated HbSS mice. Unlike in untreated HbSS mice, microvascular blood flow and FCD returned to baseline values after 72 hours of reoxygenation in treated HbSS mice. These results suggest that by increasing HbS-O2 affinity and thereby inhibiting HbS polymerization, GBT021601 may attenuate hypoxia-induced vaso-occlusion and impaired capillary perfusion. Furthermore, GBT021601 treatment in HbSS mice reduced both circulating adhesion markers and leukocyte-endothelium interactions in venules after hypoxia and reoxygenation.
Conclusions: GBT021601 increased microvascular perfusion, reduced vaso-occlusion, and improved the hypoxia-induced reduction in capillary perfusion in HbSS mice, suggesting that chronic GBT021601 treatment prevents SCD-induced microvascular dysfunction and mitigates the rebound vaso-occlusion induced by hypoxia and reoxygenation in a murine model of SCD.
Funding: Global Blood Therapeutics.
Disclosures
Dufu:Global Blood Therapeutics: Current Employment, Current equity holder in publicly-traded company. Oksenberg:Global Blood Therapeutics: Current equity holder in publicly-traded company, Ended employment in the past 24 months. Cathers:Global Blood Therapeutics: Current Employment, Current equity holder in publicly-traded company. Munoz:Global Blood Therapeutics: Research Funding. Muller:Global Blood Therapeutics: Research Funding. Pedro Cabrales:Global Blood Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal